Genetics and vascular malformations
A practical explanation of genetics behind vascular malformations
Vascular malformations are already present at birth but are not always immediately detected. They normally develop proportionally to the patient. Symptoms of vascular malformations can occur and be noticed at birth, during childhood or even into adulthood. But why do these malformations arise? They are caused by a genetic change in the DNA of the embryo during pregnancy. This is also known as a mutation or pathogenesis. This genetic change does not always occur throughout the body, but can also occur in one part of the body or in several places, depending on when it occurs during embryogenesis (Figure 1).
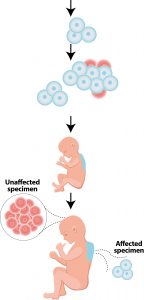
Figure 1: The embryo develops in the first seven weeks after fertilization. Cells divide to form this embryo. During this process, somatic mutations can arise, which can lead to the development of vascular malformations, skin or bone abnormalities or even brain damage.
The genetic change in the DNA of the child occurs spontaneously. This means that the parents do not carry the mutation, although rare cases of families with vascular malformations have been recorded. Because these mutations usually develop spontaneously, nothing can be done to prevent them. In addition, these mutations are not present in the germline and cannot be inherited (this is called a somatic mutation). In the exceptional case of heredity, a gene (a mutation of the germline) is passed on, which increases the risk of developing vascular malformations. There is great variability in the observed phenotype, with differences in injury severity and age at onset despite the same germline mutation being present. The phenotype depends on when this second mutation occurs, just as with the spontaneously generated vascular malformations.
DNA and genetic changes
DNA is the abbreviation of deoxyribonucleic acid, or deoxyribonucleic acid, and is the genetic material in humans and almost all other organisms. Most DNA is located in the cell nucleus and on the chromosomes (Figure 2). The hereditary information is stored in these chromosomes and has a code that consists of four nitrogenous bases: adenine (A), guanine (G), cytosine (C), and thymine (T). These bases are in pairs and together form a double helix. The code, or the way the base pairs are arranged, has the function of coding, for example, the color of eyes, growth factors, but also proteins. The slightest change in this DNA can lead to a different function of the code. In vascular development, such a small change during embryogenesis can lead to the development of vascular malformations.
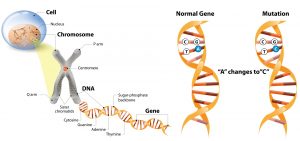
Figure 2: Schematic representation: the cell nucleus is located in the cell. The cell nucleus contains chromosomes. These chromosomes contain DNA. The DNA forms a double helix through the base pair bonds. A single change in the base pairs can lead to a functional change and is called a mutation.
Genetic changes and treatment
Since the discovery of the first somatic mutations in a vascular malformation more than a decade ago, it has become clear that there are different types of mutations in two important intercellular connections located in the cell. These connections are, as it were, the guideline for the functioning of a cell in which a group of molecules works together. The human body contains a large number of these intercellular connections, but in the case of vascular malformations, two of them prove to be extremely important. These are the RAS/MAPK/ERK compound and the PIK3 (phosphatidylinositol 3-kinase)/protein kinase B/mTOR (mammalian target of rapamycin) compound. Different types of mutations can be detected within these compounds, which lead to the development of vascular malformations. Mutations in the RAS/MAPK/ERK junction are found in patients with arteriovenous malformations, while mutations in the PIK3CA/mTOR junction are more commonly seen in patients with venous or lymphatic malformations and patients with PROS syndrome, which causes overgrowth. While these mutations can be different, all can lead to hyperactivation of one of the compounds, causing, for example, the growth of aberrant blood vessels. When it is known which compound is active, or which mutation is the cause of the activation, a more targeted medical treatment can be initiated. For example, rapamycin, also called sirolimus, can inhibit the mTOR compound. In addition, new agents such as alpelisib show promise for patients with a PIK3CA mutation.
Author Dr. Maroeska te Loo, pediatric hematologist and clinical pharmacologist
HECOVAN working group, Radboudumc, Nijmegen, Netherlands





