The European organisation for rare diseases Eurordis organised a members conference in Bucharest (Romania) from 16 to 19 May.
On behalf of the CMTC-OVM organisation, the chairman Lex van der Heijden participated in this conference. Around 270 people attended this conference.
First day
The first day was meant to bring together European Patient Advocates Groups (ePAGs) to exchange knowledge and experience. The subject of ‘developing patient journeys’ appealed the most.
Johan de Graaf: registerproject (EuRReCa)
EuRReCa is a very powerful tool to give all patients, healthcare professionals and researchers the opportunity to participate and use high-quality, patient-oriented registers for rare endocrine (hormonal) disorders that fall under Endo-ERN (Expert Reference Network, ERN). EuRRECa is closely linked to Endo-ERN, but is open to all professionals who provide endocrine care and who want to participate.
During the development they organised a ‘Patient, parent and ethical’ working group. In the future, the ‘Patient, parent and ethical’ working group will have to give approval in order to register.
Data can be collected by patients or clinicians. Ideally both. Registration was both technical and about the quality of life.
Patients must be involved in all stages. The core of a register is a limited data set. The data to be used must come from both patients and clinicians.
Registries must be FAIR (Findability, Accessibility, Interoperability and Reusability). The ownership of registers may not be clear yet.
Permission forms have been developed that can be used by all countries in all hospitals.
Russel Wheeler, Rare Eyes Disease (RED) Ontology using the Orphanet and HPO database
ERN-EYE recognised at an early stage that in order to allow cooperation between geographical and professional boundaries, there was a clear need for a common language for use, not only in research and registers but also in the presentation of cases for consultation. A workshop was organised to review two of the most important existing ontologies to ensure they were fit for purpose.
The first thought was that since this is a highly technical exercise, patient involvement would be a distraction and that non-scientists would not be able to participate in such high-level debates.
We are all impatient patients. Read, search, be curious and study.
Hospitals use different codes for the identification of diseases: Orphanet has a code based on genetics; Human Phenotype Ontology has a coding based on the diagnosis. You need permission about the coding and name of the rare disease to translate it into other languages and apply it in the Clinical Patient Management System (CPMS). A consensus document was signed in this study.
Conclusion for registration there are three important points:
- We, the patients, have a voice that should be in a register.
- Long-term data also entails short-term data. Ask people to stay for long-term data, but publish short-term data to motivate them to get involved in registration.
- Train and communicate to show the benefit of registration and to eliminate the fear of GDPR.
Ilaria Galetti, Patient involvement in identifying unmet needs on clinical guidelines for patients
For every illness covered by ReConnet, an advocate of ePAG has been appointed in ReCONNET. The diseases are subdivided into 3 disease groups where an advocate of ePAG per group has been appointed as a ReCONNET Steering Committee (SC) member.
The SC decided to publish a descriptive review of the existing Clinical Practice Guidelines (CPG) in order to perform a state-of-the-art of the existing Clinical Practice Guidelines (CPGs) per disease group. As a result of a proposal from the ePAGs in ReConnet SC, clinicians agreed to identify the needs of the unfulfilled patients for each disease as part of this exercise. It was then decided to have doctors and patients prepare the papers for each illness; the ePAG patient advocates a contribution aimed at collecting and writing the unmet needs of each disease by the patient. Several advocates of ePAG patients were involved in this exercise and their contribution as newspaper co-authors was fully recognized.
Patients had a positive influence on the published articles. Their unmet needs are recognised by healthcare professionals and will ultimately result in new projects.
Clinicians and patients develop guidelines together. There is usually a chairman, a group of medical experts and a patient when drafting guidelines. To be part of this group, you must have some information about the process and understand the methods (such as Delphi research). Patients work for free. Ilaria asked national patient care groups for anamneses. Patient representative in guideline was named as co-author.
Guidelines were translated into comprehensible language. When simplifying the text, patients were asked which words were still too difficult. To simplify, images can also be used. The next step was to translate them into different languages. The translation of the guidelines is done by European society free of charge. No money was available.
They prepared 15 universal questions that will help patients look for information. To answer the questions, they used 4 guidelines. An example of a question might be: “Do we have enough nurses for the quality of life?”
All European countries were involved in this ERN study, some do not even have a plan for rare diseases.
Develop guidelines: problem: patients work for free. Patients were named as co-authors.
Guidelines have been translated into understandable language (simplified). As soon as a patient version was available, she asked patients which words were still too difficult. Photos were added to the patient version.
The patient version of the guidelines then had to be translated into different languages. She had involved all European countries, even those that do not yet have a plan for rare diseases. The language translations were done by the European community, but no money was available.
Sue Routledge and Ammi Sundqvist, develop Patient Journeys
The information in the table is summarised in a visual “Patient Journey” or “roadmap” to support user-friendly communication. During a personal workshop with the ERN coordination team, every ePAG advocate presented their patient journey, their life path from the first symptom, from the diagnosis to care, treatment and follow-up. This was presented to the wider ePAG group to support a better understanding of each other’s diagnosis. In between each presentation, the group was asked to highlight common needs that others had experienced in their rare disease. The common needs for all ERN ITHACA syndromes were recorded through this workshop and summarized in one common patient journey.
The next step was to establish recommendations for each of the identified common needs and for the ERN to address how the network will address these common needs both at the strategic and operational level. One of the main concerns we had with this exercise was how we could represent the journey of other diagnostic groups within ERN ITHACA. We raised the concerns we had with Matt Bolz-Johnson and he created a template to describe a journey of patients across the various diseases. To summarise our needs, we wrote down how we think our own journey should be in healthcare by completing the template.
They have developed patient travel for patients (parents) to find out more about the implications because the diagnosis of complications is often too late. It was useful to identify the needs of clinicians. Sometimes the impact of information can be frightening, so awareness is necessary – although a Google search may provide information that is much worse!
Second day
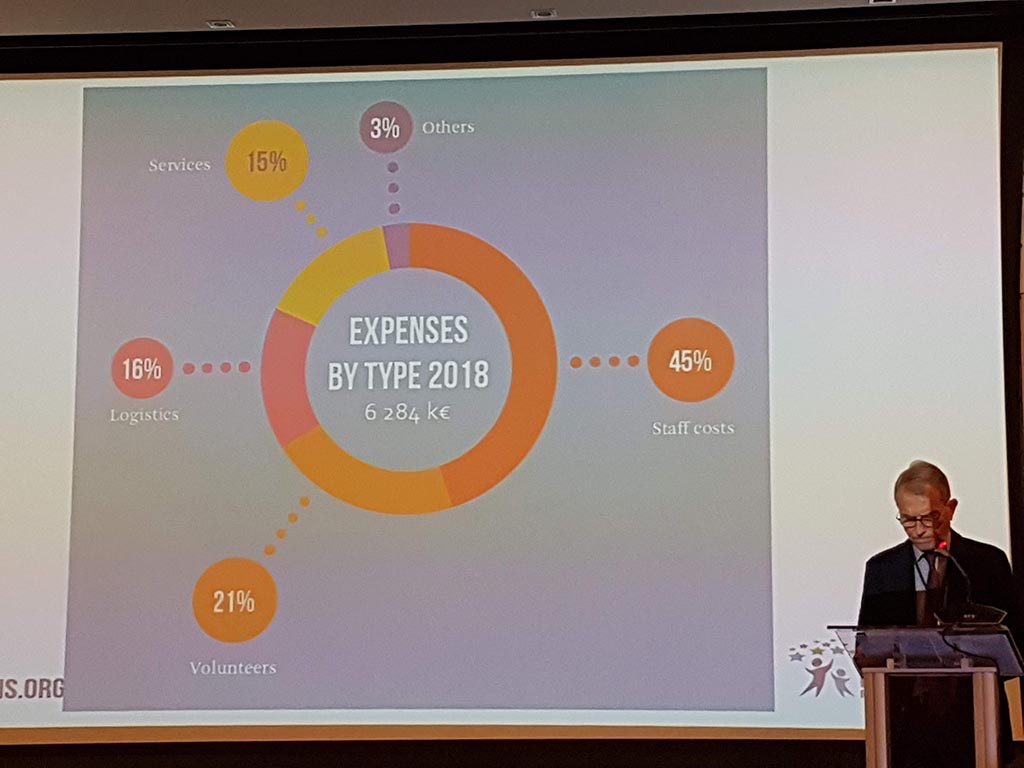
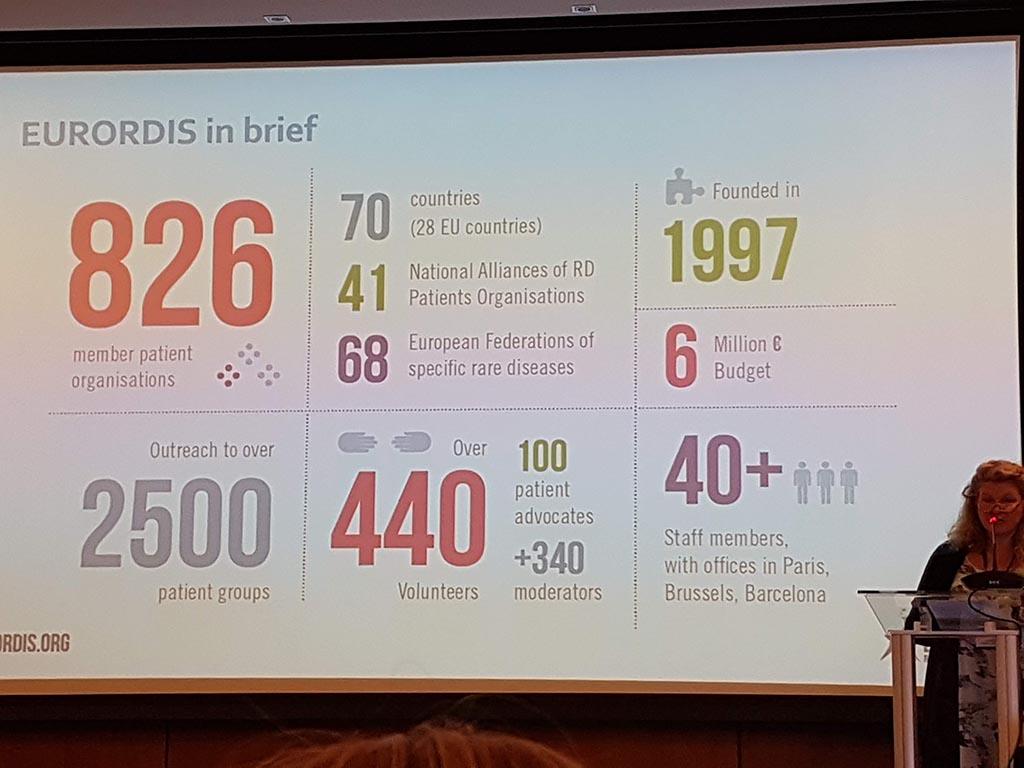
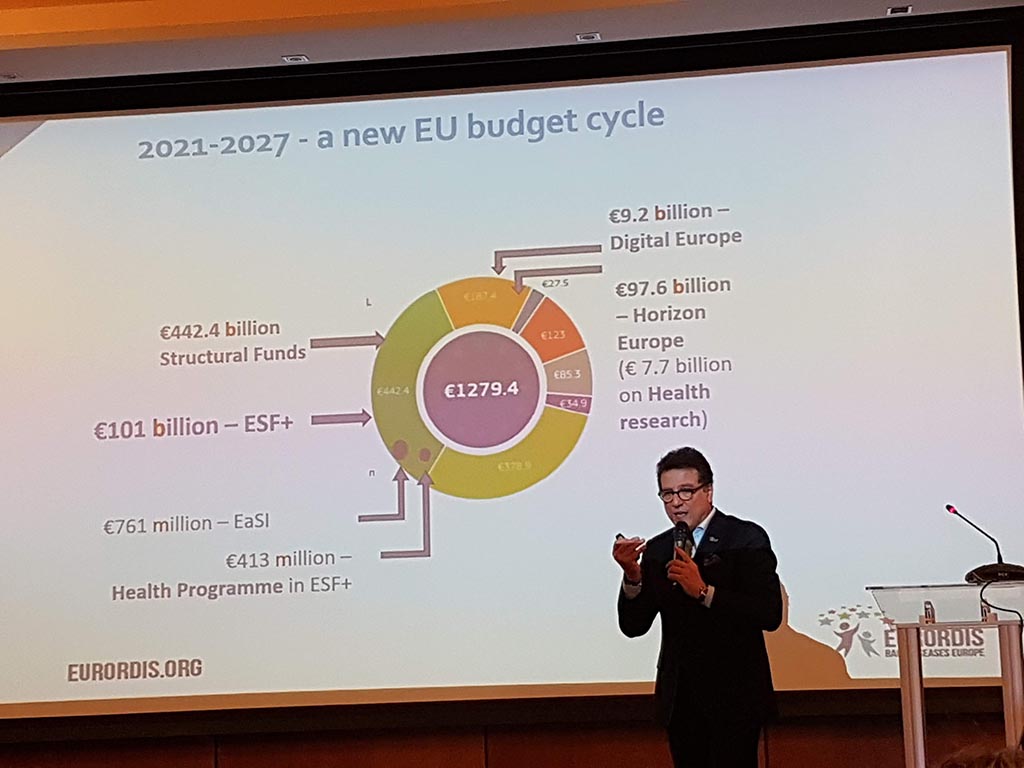
The second day started with the members meeting during which the annual report (activities and finances carried out) were discussed. Eurordis has put rare diseases on the map at both the United Nations and the World Health Organization!
Subsequently, new members were elected to the Board of Directors.
Yann Le Camm gave an excellent overview of the activities of the year 2019 and the strategy.
After lunch several other people spoke, such as a representative from the Ministry of Health and Princes Maria was present at the Royal Family.
Third day
This day consisted of all kinds of workshops:
- How to make the best use of the Eurordis ‘position paper’ about holistic care.
- Why set up a community advisory board.
- European Reference Networks – the Eastern European countries dimension.
- Contributing to the following 10 year rare diseases policy: rare 2030 forward-looking study.
- Sharing and protecting our health data.