On 20, 21, and 22 October the American Organization for Rare Disorders NORD organised another annual congress with around 900 participants.
On Sunday afternoon a special session was organised for the Patient Leaders. We were placed into groups and a specific subject was discussed per table. Lex participated in the discussion on good governance and board management.
The conference on Monday and Tuesday consisted mainly of panel discussions. The panels consisted of a wide variety of participants with different backgrounds such as patient organisations, the US Food & Drug Administration (FDA), pharmaceutical companies, National Institute of Health (NIH) and NORD.
Some notable points:
- Science makes great progress and more and more companies are stepping into the world of the rare disease.
- What one is concerned about: the American elections in 2020, the high prices of medicines and misunderstandings surrounding the Orphan Drug Act (orphan drugs).
- For years, people have been talking about the lack of transparency about the cost of medications.
- The intermediary trade will cover approximately 40% of the costs.
- Patients need fair-priced medications and good access to medications.
- The price of medicine should be based on the value and not on the risk.
- More attention should be given to what patients deem important, not only reported outcomes.
Discussion points at the panel on the use of social media:
- Facebook is the most widely used platform.
- The most abused information is information that leads to a person, credit card information and health information.
- In the USA, the health insurance number is most abused. In Europe this is the citizen service number and date of birth.
- Pay attention to who is following you on social media and how often you can log in to an account before it is blocked.
- As an organisation, you have to pay attention to volunteers. When do they come in, they leave the organisation again and what data do they have/have access to?
The following panel discussion was titled ‘ A Unique Moment in Time for Rare Disease Research ‘: Industry, government, science and patients work more closely together on the same goals than ever. For decades we have searched for how we can connect with each other. It seems that we now have the same goals and mission. Becoming a greater whole than the sum of its parts.
Alex Azar, secretary of Health and Human Services Secretary gives a presentation about the Trump government’s vision on health care:
- Protect what works and fix what doesn’t work.
- Reduce costs, accessible health care and improve health in rare diseases.
- He wants to investigate how to cope with the high cost of therapy in Medicare and private health insurance costs.
- The cost of medications should be transparent and the cost should be lower. He thinks that competition will lower the prices of medicine.
- Affordable and personal patient centred healthcare is a goal of this government.
- How healthcare is now being funded needs to be reformed.
- This government is committed to protecting individuals with pre-existing conditions.
Panel with FDA Center Directors
- Doctors and patients often think differently about the burden of disease and the burden of using medications.
- People should not take medications so that other people feel better.
- Patient Advocacy is important. The FDA would like to involve more patient organisations in the whole.
- The FDA would like to work with groups of broad concepts rather than specific medications.
- The need for patient advocacy is becoming increasingly important. Handle an agenda with the important aspects of the condition, possible outcomes and how to collect data. Pay attention to the burden of disease and the burden of treatment.
- The FDA is beginning to think of applying personalised medications in rare conditions. We are only years away from this. The medications may not be commercially feasible. Currently, more than 800 gene therapies are under development. The FDA is banned from talking about specific medications that are being reviewed without the producer’s permission. Sustainable production is crucial.
- We are considering the creation of a production consortium to enable individual therapies.
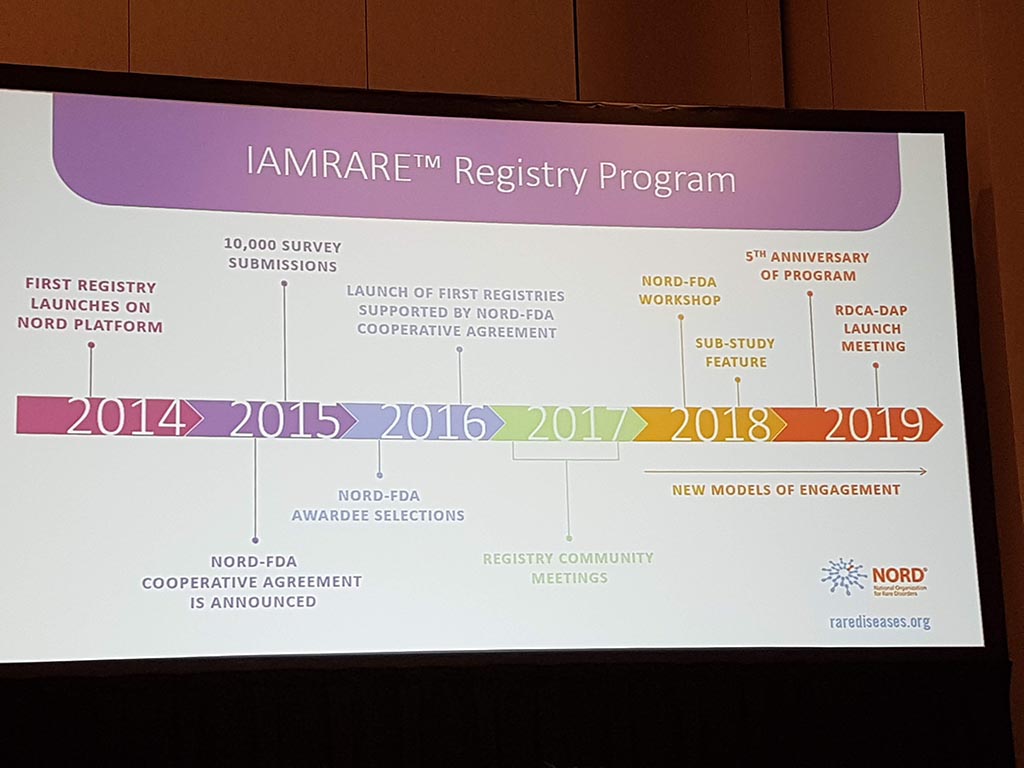
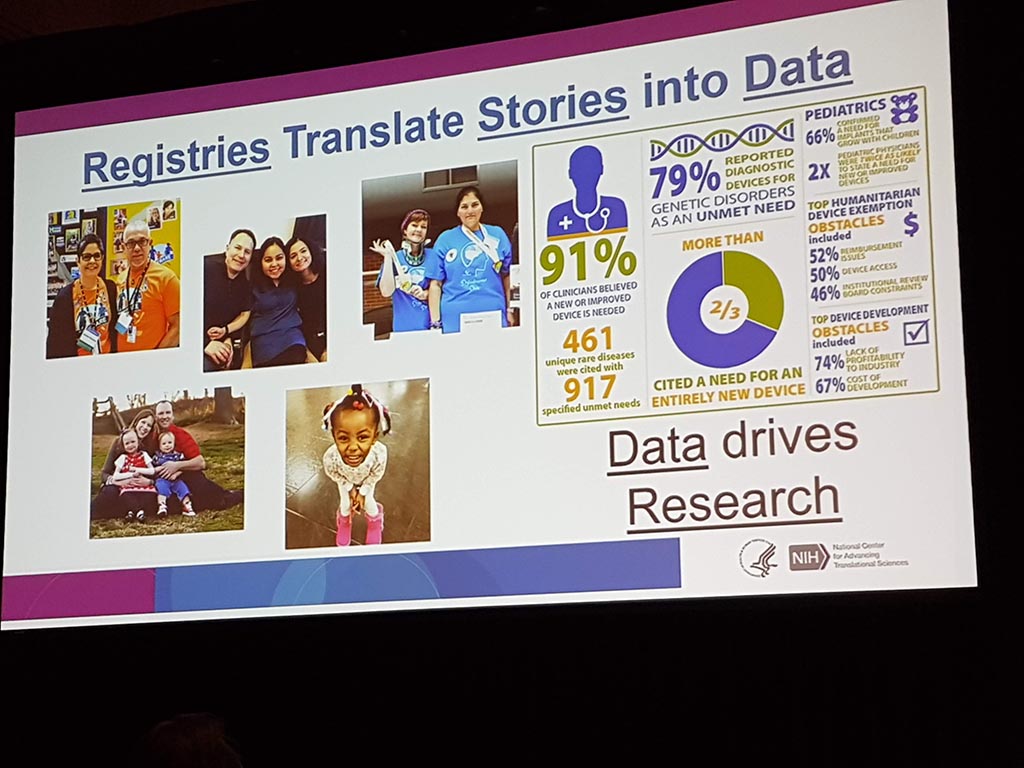
Patient Registries and Natural History Studies: Impact, Data Ownership and Ethical Issues.
For many years there has been talk of ‘patient registries’ where medical data of patients in particular are stored. Such registries are a way to translate stories from many individual patients into data. Such registers are of great value, for example, in research into a specific condition. Given the highly sensitive medical data, a lot of attention needs to be paid to privacy and security!
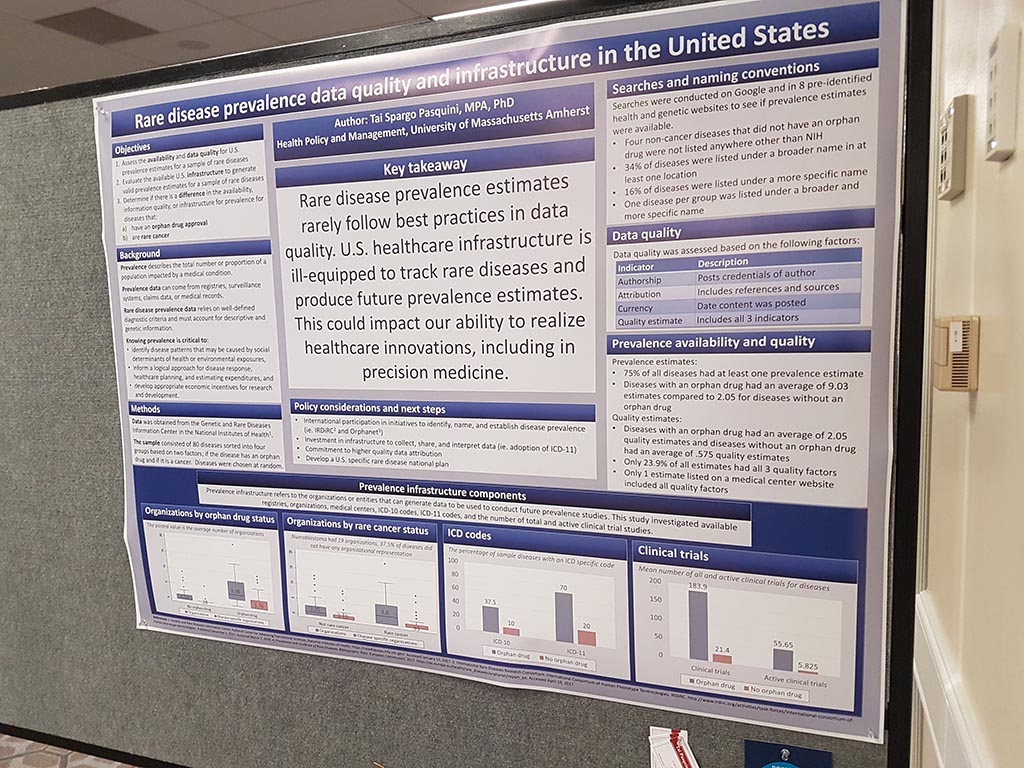
Every year, NORD also organises a ‘poster session’ in which organisations can present themselves. In the space, where all sorts of other organisations and companies have a booth and where lunch is served, there are signs prepared on which a poster can be attached. This year we participated in this poster session with a new poster.
What have we achieved during this conference:
- New contacts with other patient organisations. Experience shows that this often does not directly produce a measurable result. One of the new contacts is with a board member of the Chinese organisation for rare diseases.
- Firstly, they help us to translate leaflets into Chinese and, secondly, to reach out to patients and families in China.
- Deepening of contacts with NORD. The majority of the NORD staff knows our organisations by now. As soon as they see something passing in areas such as CMTC and other vascular malformations, our organisation is contacted.
- Thanks to our NORD membership, we can use their social media channels, newsletter, etc. to profile our organisation better and make it more visible. Because we know each other personally, we can switch quickly.
Also thanks to Marjolein van Kessel.