The Canadian Organisation for Rare Diseases (CORD) organised a conference in Toronto from the 10th through the 12th of May, 2019.
Lex participated in this conference on behalf of our organisation.
Approximately 140 people were present from countries from all over the world. In addition, again meeting with the old acquaintances, new contacts were made, for example, with representatives of rare disease organisations from China and Taiwan.
We have known Christina Mutena, from Kenya (Africa), for more than 10 years and we finally met! She is our patient advocate in Kenya.
Lex had, of course, again brought stroopwafel and chocolate! The reactions were great! Not everyone knows what a stroopwafel is (yet) but after having tasted it…
Below are some short reports from various speakers.
Peter Goodhand
Peter Goodhand (CEO Ontario Institute for Cancer Research) described that there are worldwide approximately 350 million people with rare diseases. For example, rare types of cancer are also included. One of the biggest problems is making the correct diagnosis, which often takes too long. He argues that researchers should share their knowledge, experience, and also genetic information. He also argues that working together, physically coming together, and travelling are crucial when it comes to rare diseases.
Chris McMaster
Chris McMaster (scientific director CIHR Institute of genetics) experiences that in hospitals there is a lack of data on rare diseases. How many workers in a hospital has knowledge of rare diseases what the costs are, how many dead are there, etc.? He believes that the problem is that one does not know how to measure these factors. He argues that the greatest costs in a hospital are related to the care systems.
Sébastien Martel
Sébastien Martel (global head of rare diseases Sanofi) indicates there is no cure for 90% of rare diseases. Patient organisations are always better organised and the ‘ patient advocates ‘ are very effective. He would like to have patients be monitored for long periods so that one can learn from this.
David Malkin
David Malkin (the Hospital for Sick Children in Toronto) advises working in multidisciplinary teams in multiple institutes when it comes to rare diseases. For adults, medication is often not a problem. Also, it cannot be assumed that adult medication works the same in children, but also, due to regulations, access to medication for children is much more difficult. David thinks that the price of medicines should be considerably reduced.
Garteh Baynam
Garteh Baynam (Western Australia Health Department, well-known to us), noted, among other things, that there are relatively few inhabitants in Australia and also with a low population density. One of the problems he sees is the difficulty in setting up a sustainable health system in Australia. In 2018, a start was made in establishing a framework for rare diseases with the following strategic priorities: diagnosis, access to treatment, data collection, coordinated care, access to services and coordinated research.
Michael Liebman
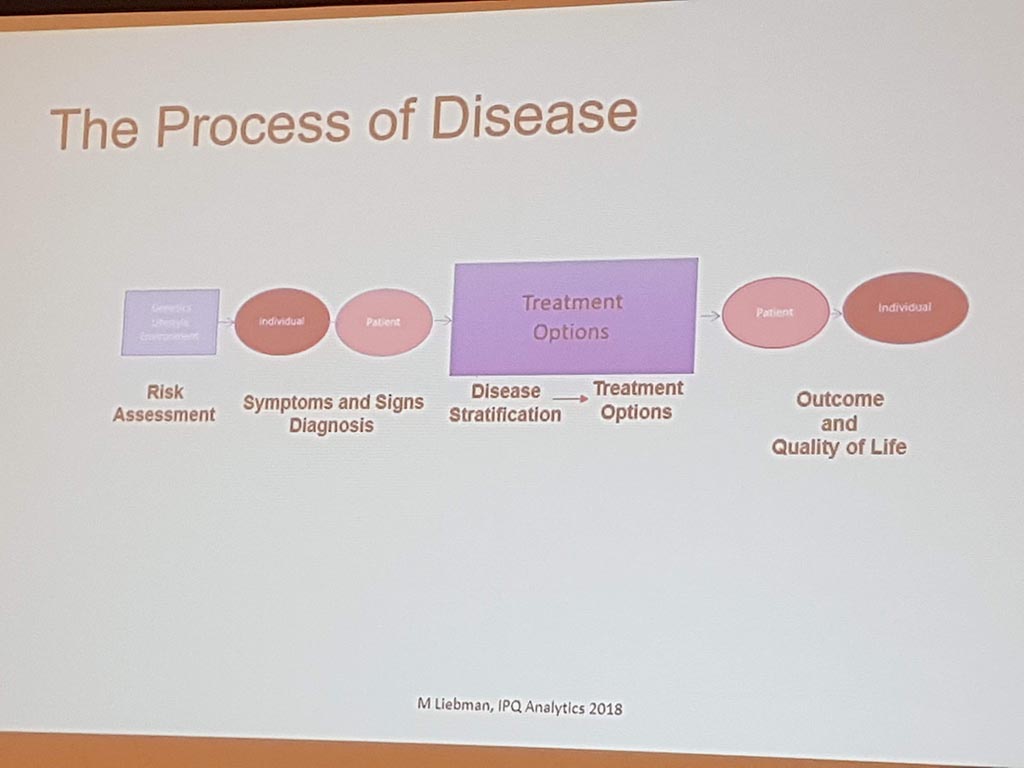
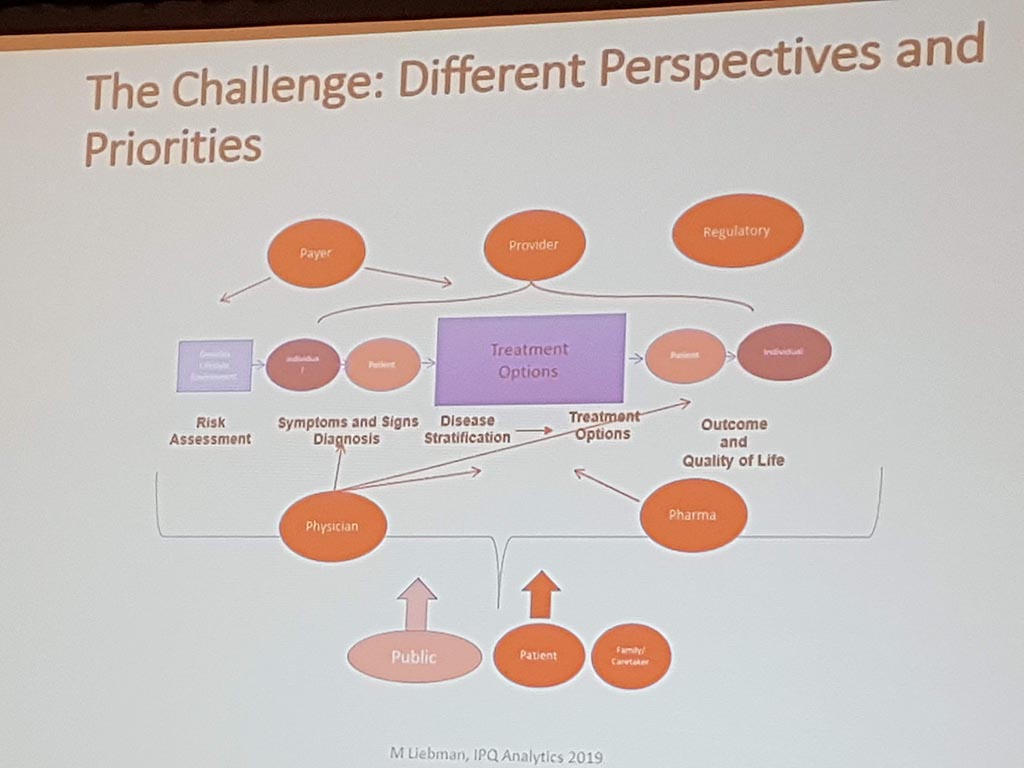
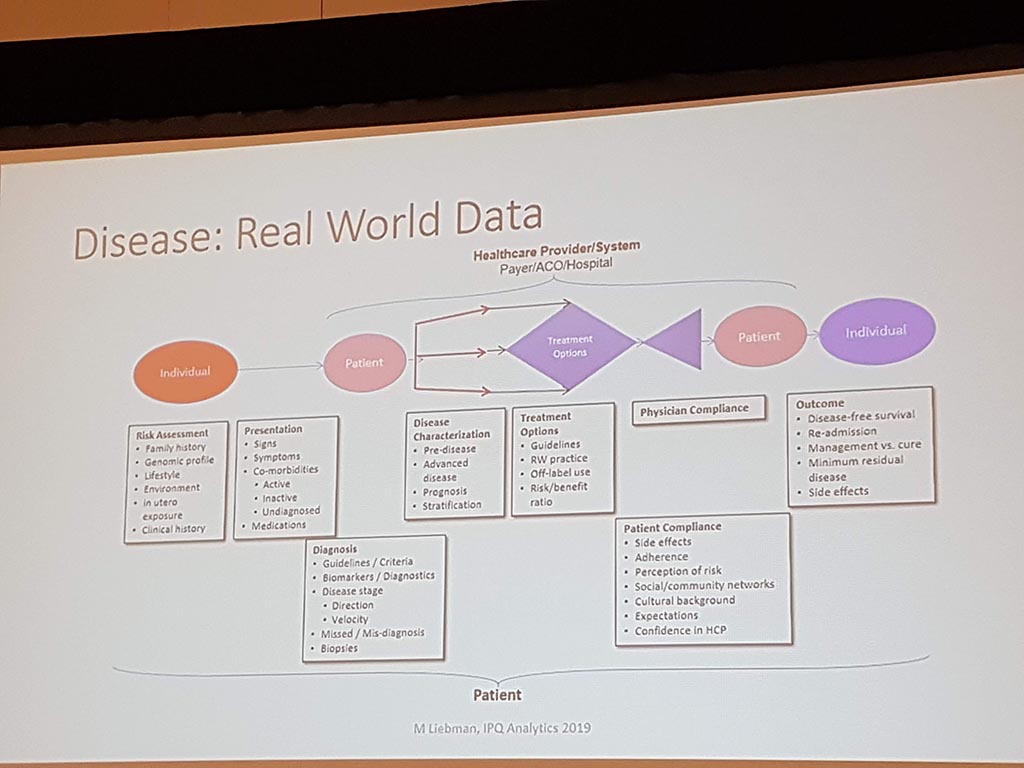
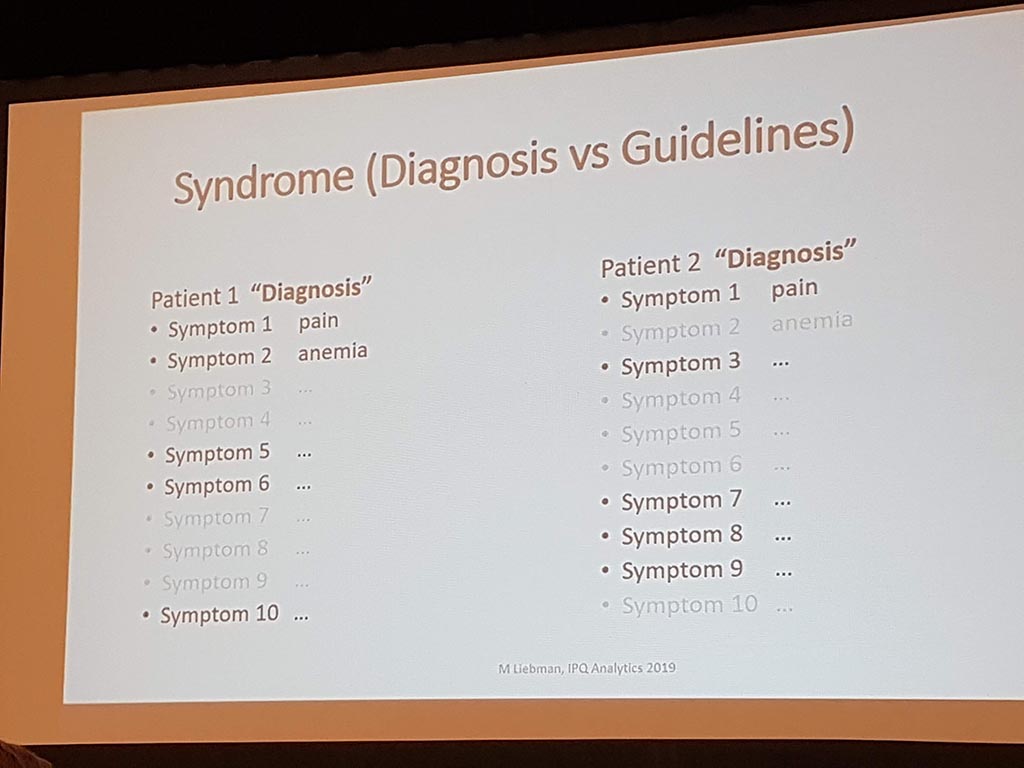
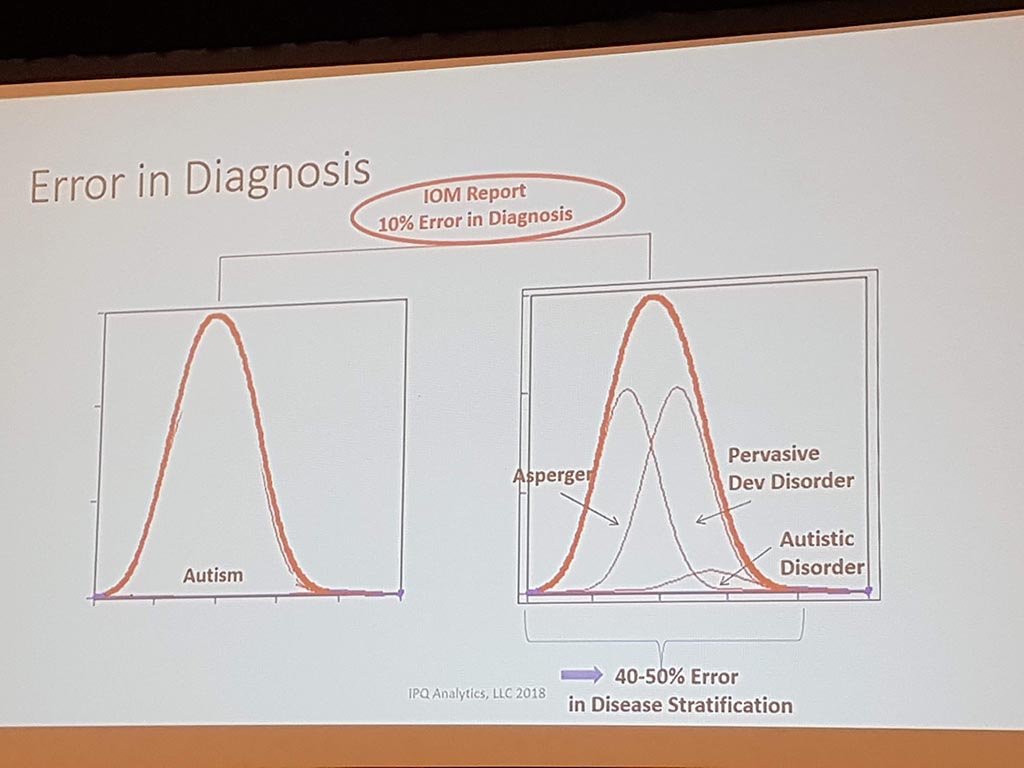
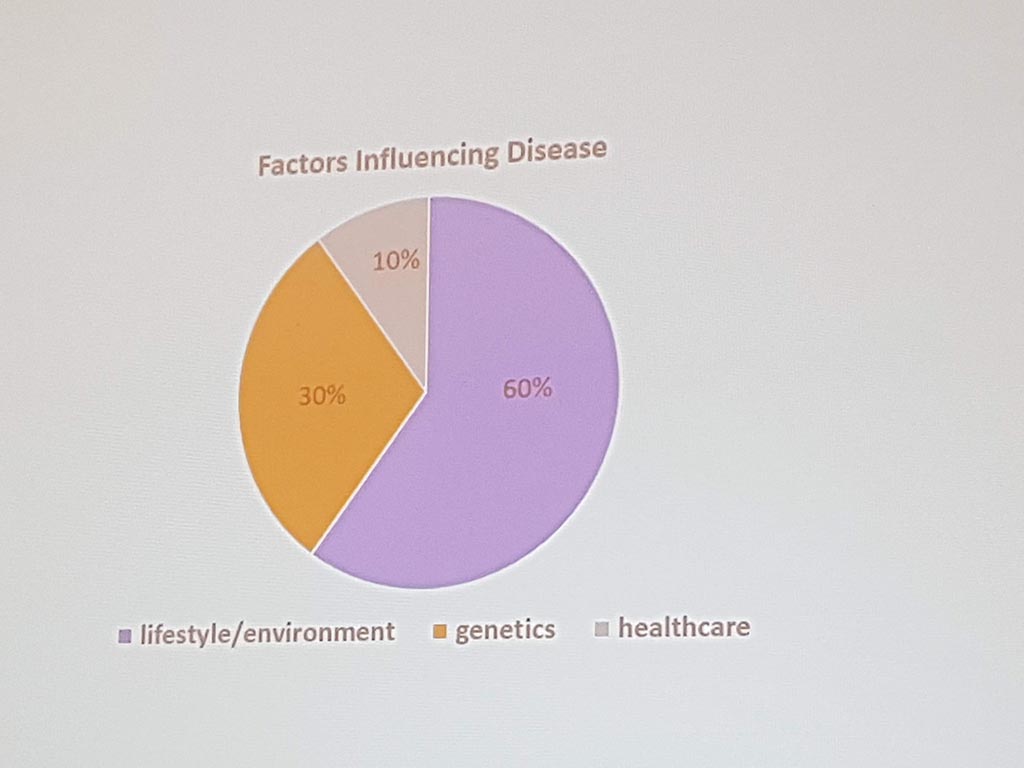
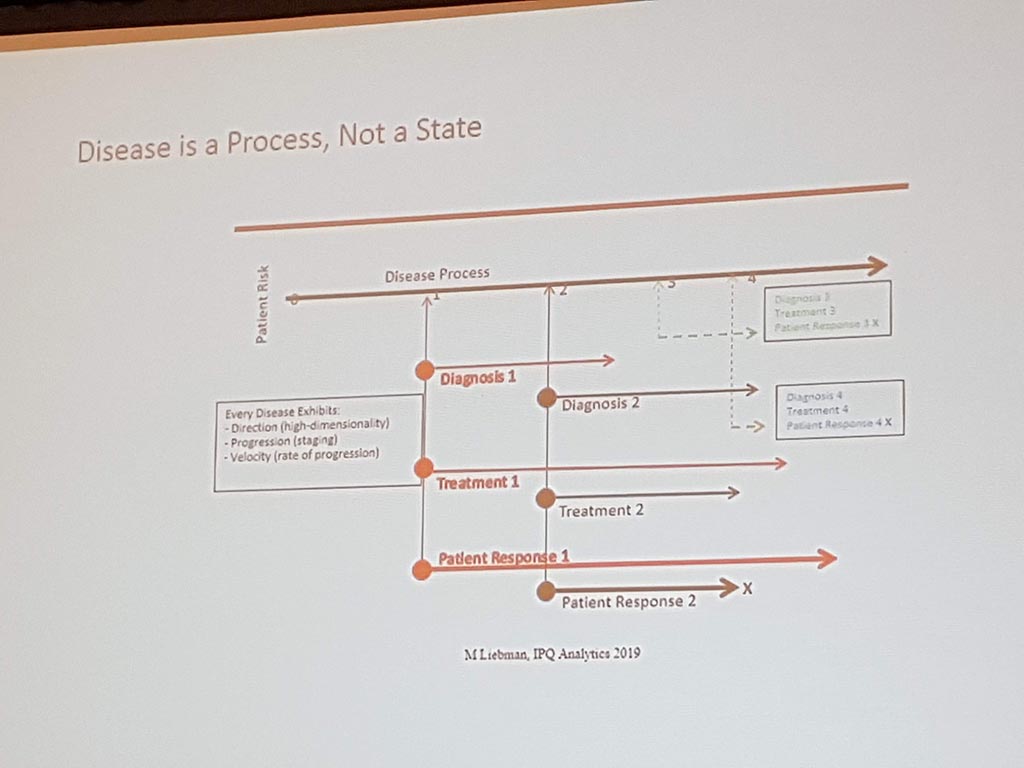
Michael Liebman (managing director ipq Analysis) started with the description of the disease process. His conclusion is that ‘ real ‘ patients with a disease are complex. he considers the struggle between different perspectives and priorities challenging. For many diseases a checklist was made with criteria that a patient must ‘ meet ‘ in order to be diagnosed with a particular disease. A minimum of 5 criteria must be met. However, for different patients, there may be different criteria. This can result in an incorrect diagnosis. In a study by Singh in 2014, it was shown that at least 1 out of 20 adults in the USA are incorrectly diagnosed. This amounts to 12 million false diagnoses in the USA, where approximately 50% cause potential harm to a patient. He also gave as example autism where 40-50% of the diagnosis are incorrect. What he also described the relationship between lifestyle and environment to rare diseases. Factors affecting the disease are: 60% lifestyle/environment, 30% genetic and 10% health care. His thesis is that disease is a process and not a status.
Hartmann Wellhofer
Hartmann Wellhofer (Takeda Pharmaceuticals) started his presentation with the thesis that 82% of patients with Fabry get no diagnosis or a wrong diagnosis. His vision is that progress in the field of rare diseases is driven by quicker diagnosis, cooperation in setting care standards, development of new therapies, and artificial intelligence and ‘ Big Data ‘. He also believes that cooperation with patients and ‘ patient advocates ‘ is crucial.
What I noticed is that in Europe there is a lot going on in the field of rare diseases. The European Organisation for rare Diseases, Eurordis, plays a leading role in this. They are working intensively with the European Union and the European Commission. Yann Le Cam, the CEO of Eurordis, is the driving force behind this.
Meanwhile, rare diseases within the United Nations and the World Health Organisation (WHO) are now being recognised and this has a significant impact on the benefits for those with rare diseases
Tori Lacey
Tori Lacey (Canada) gave a concise view of what she said to be the most painful issues for a patient with a rare disease. She has spinal muscular atrophy and requires a wheelchair. This results, according to her, in the loss of independence and dignity.
Debby Lambert
Debby Lambert (orphanet Ireland) gave a presentation with statistics related to rare disease. These figures are used by ‘patient advocates ‘, governments and policymakers, researchers, and pharmaceutical companies. The number of unique rare diseases is currently (May 2019) 6172. 86% of these diseases are long-term or chronic diseases and 14% are caused by, for example, cancer, infections, and poisonings. 70% of the cases involve young children, and 12% adults, and by 18% in young children or adults. 72% of rare diseases have a genetic origin.
Peter Jones
Peter Jones (Microsoft Canada) described a project that Microsoft is doing in collaboration with Eurordis and Takeda (pharmaceutical company), with the aim of ending the problems involved in medical diagnosis (they take too long and often result in incorrect diagnosis). The main focus is on centres of excellence, genetic screening, data sharing and privacy.